Speakers and Talks
Speakers and Talks
Translating Cell and Tissue Engineered Therapies
Tuesday, February 25, 2:50 - 3:30 PM
In-person talk: Gordana Vunjak-Novakovic (Columbia)
Recorded talks:
- Rhiannon David(Astrazenaca/IQ-MPS Affiliate)
- Richard McFarland (BioFabUSA)
Dr Rhiannon David is Director, Microphysiological Systems (MPS) in Clinical Pharmacology and Safety Sciences at AstraZeneca, Cambridge, UK. Rhiannon is responsible for devising and delivering a strategic plan for the development and integration of advanced cell models, including spheroids and MPS, to achieve quantitative human-translation of pre-clinical safety assessment with 3Rs impact. She is Chair of the IQ MPS Affiliate and a member of several advisory boards, including the Industrial Advisory Board for the European Organ-on-a-Chip Society (EUROoCS).
Richard McFarland, Ph.D., MD is the Chief Regulatory Officer Chief Regulatory Officer of ARMI/BioFabUSA. Prior to ARMI he was Associate Director of Policy for FDA/CBER’s Office of Tissues and Advanced Therapies and its predecessor office, the Office of Cellular, Tissue and Gene Therapies. In this position, he was heavily involved in policy development for tissue engineering, regenerative medicine, and alternatives to animal use in regulatory decision making. Dr. McFarland received his B.S., Ph.D., and M.D. from the University of North Carolina at Chapel Hill, and completed his anatomic/clinical pathology residency and immunopathology fellowship training at UT Southwestern in Dallas.
Intro to the Advanced Regenerative Manufacturing Institute (ARMI) - Recorded Talk
Tuesday, February 26, 1:20 - 1:50 PM
The Advanced Regenerative Manufacturing Institute (ARMI) is a member-based, nonprofit organization whose mission is to advance the bioeconomy of the United States. Dr. McFarland will introduce a number of programs ARMI provides including BioFabUSA, ARMI BioIndustries, and NextFab.
 (1).jpeg)
Bio for Richard :
Richard McFarland, Ph.D., MD is the Chief Regulatory Officer Chief Regulatory Officer of ARMI/BioFabUSA. Prior to ARMI he was Associate Director of Policy for FDA/CBER’s Office of Tissues and Advanced Therapies and its predecessor office, the Office of Cellular, Tissue and Gene Therapies. In this position, he was heavily involved in policy development for tissue engineering, regenerative medicine, and alternatives to animal use in regulatory decision making. Dr. McFarland received his B.S., Ph.D., and M.D. from the University of North Carolina at Chapel Hill, and completed his anatomic/clinical pathology residency and immunopathology fellowship training at UT Southwestern in Dallas.

Bio of Rhiannon :
Dr Rhiannon David is Director, Microphysiological Systems (MPS) in Clinical Pharmacology and Safety Sciences at AstraZeneca, Cambridge, UK. Rhiannon is responsible for devising and delivering a strategic plan for the development and integration of advanced cell models, including spheroids and MPS, to achieve quantitative human-translation of pre-clinical safety assessment with 3Rs impact. She is Chair of the IQ MPS Affiliate and a member of several advisory boards, including the Industrial Advisory Board for the European Organ-on-a-Chip Society (EUROoCS).
JI Session - Cancer TEC Trainees: Looking Back and Looking Forward
Tuesday, February 25, 2:30 - 3:20 PM (Bascom Palmer Eye Institute Executive Board Room #252)
Chair: Kwaghtaver Samuel Desongu (Auburn) and Hunain Khawaja (University of Arizona)
Bascom Palmer Eye Institute Boardroom Room #252
This session provides an opportunity for trainees (graduate students and postdocs) to engage in meaningful discussion regarding career goals, research interests, trainee specific issues, and broadly trainee engagement. During the session, trainees will discuss recent opportunities available for trainees as part of the Cancer TEC group and how best to pursue them. We would like to receive feedback on trainee programs held in the past, better understand the needs of trainees, and open dialogue on how to meet these. If you are a trainee, please come and don’t be left out.
Breakout Sessions
- 1 - Biomaterials & TME (Lead: Kris Kilian) - Jose Berrocal Auditorium
- 2 - Metastasis (Lead: Luis Solorio, Shannon Mumenthaler, Joanna Burdette, Mike Wendt) - McKnight Vision Research Center 1st Floor Room #102
- 3 - Drug Screening and Discovery (Lead: Xiling Shen & Meenakshi Upreti) - McKnight Vision Research Center 1st Floor Room #111
Biomaterials & TME Breakout Session
Chair: Kris Killian
This session aims to bring together junior and senior investigators to discuss topics in biomaterials science and engineering as it relates to modelling the tumor microenvironment (TME). Discussion will be centred around key questions and challenges in the field within the context of studying complex TMEs and mimicking these attributes using biomaterials to aid biological discovery and individualized treatments.
Metastasis Breakout Session
Chairs: Luis Solorio, Shannon Mumenthaler, Joanna Burdette, Mike Wendt
The goals of this breakout session are to provide a framework to articulate challenges in both the technology and biology space, as well as to provide an opportunity to initiate new collaborations. Some of the topics that may come up are tumor dormancy, cellular plasticity/heterogeneity, models of matrix remodeling, and models of invasion. More details TBD.
Drug Screening and Discovery: 3D Biology in Drug Development and Precision Medicine of Cancer: Current Advances and Expectations
Chairs: Xiling Shen & Meenakshi Upreti
This session will address some or all of the following topics:
- 1 - Selecting the most promising and safe drug candidates at the pre-clinical stage
- 2 - Overcoming challenges in 3D in vitro modelling of cancer
- 3 - Comparing the key benefits and applications of Tumor organoids alongside conventional 2D and 3D platforms
- 4 - Multiparametric analysis using 3D platforms
- 5 - Ensuring genomic and phenotypic stability and ability for long-term expansion
Scientific Sessions
Session 1 – TME
Investigating Lymphovascular Space Invasion in Inflammatory Breast Cancer Using a Vascularized 3D Microfluidic Platform
Melika Mehrabi Dehdezi, University of Texas at Austin
Highly aggressive cancers are frequently characterized by tumor cell emboli within the lymphatics and blood vasculature, a phenomenon known as lymphovascular space invasion (LVSI). LVSI is thought to represent one of the necessary events during progression from a localized to metastatic cancer. While LVSI is found commonly across cancers, its frequency in inflammatory breast cancer (IBC) makes IBC the ideal model system to study mechanisms responsible for formation and persistence of LVSI. However, the specific mechanisms enabling and promoting LVSI remain poorly understood.
In this study we utilized our vascularized in vitro 3D tumor microfluidic platform to compare different IBC cell lines in terms of cancer cell invasion into blood and lymphatic vessels and emboli formation. The platform's fabrication involved polymerization of collagen type I solution in which cancer cells were seeded around a 22G needle. Upon needle extraction, channels were formed which were seeded with either Telomerase immortalized endothelial (TIME) cells tagged with mKate for the blood vessel or Human Dermal Lymphatic Microvascular Endothelial Cells (HDLMVECs) tagged with RFP for the lymphatic vessel. Then, the platforms were exposed to a shear stress withing the physiological range using a rocker plate to establish an aligned and functional endothelium. Images were taken utilizing confocal microscope daily for one week to assess vessel sprouting, vessel permeability, emboli formation, and tumor cell intravasation. Cytokine analysis was performed on the collected media . We were able to capture IBC emboli intravasating the blood vessel and also migrating through the lymphatic. We have observed that lymphatic sprouting occurred at a slower rate compared to blood vessel sprouting. Furthermore, TIME cells showed a more elongated morphology whereas HDLMVECs exhibited a more rounded button like morphology.
Other Authors: Marissa Nichole Rylander, Wendy A. Woodward, Bisrat G. Debeb
Obesity-linked Colorectal Cancer (CRC) and Consensus Molecular Subtype 4 (CMS4) Colorectal Cancer Models
Michael Greene and Kwaghtaver S. Desongu, Auburn University
Modulation of the tumor microenvironment is known to promote the growth and survival of colorectal cancer (CRC) cells. Strong epidemiological evidence links certain types of human cancer, including CRC, with obesity. However, the impact of the obese tumor microenvironment on CRC tumor cellular composition, stiffness, and ECM composition is not known. The objective of our project is to examine modulation of the tumor microenvironment and obesity-related CRC disease progression using tissue-engineered patient derived xenograft (PDX) CRC models validated through comparison to patient tumors and PDX tumors. We will test the hypothesis that obesity alters stromal signaling in the consensus molecular subtype 4 (CMS4) tumor microenvironment. To test our hypothesis, we have developed a tissue-engineered platform that enables long-term in vitro culture of the patient-derived CRC cells and recapitulation of the native tumor microenvironment, particularly the stromal component. In Specific Aim 1 we examined the extent to which 3D in vitro engineered CMS4-derived CRC tissues recapitulate patient and PDX tumors. We have observed culture time-dependent recapitulation of the originating tumor characteristics in two separate CMS4 PDX lines. In Specific Aim 2, we have refined our in vitro model of inflammatory, insulin resistant adipose tissue and examined the capacity of conditioned media from inflammatory, insulin resistant adipocytes to modulate the tumor microenvironment in 3D in vitro engineered HT-29 cell-derived CRC tissues. Overall, we seek to develop a portable tissue-engineering platform with robust cross-validation so that this tool can be used to uncover CRC subtype specific mechanisms of obesity-driven tumor progression.
Other Authors: Elizabeth A. Lipke, Ifeoluwa Odeniyi, Elham Seyyedi Zadeh, Jannatul F. Nipa, Kathryn Edmondson, and Camp Jernigan
Breast Tumor Microenvironment and Metabolic Reprogramming Promotes Innervation
Nikolas Ala-Kokko, University of Arkansas
Breast cancer is the most diagnosed form of cancer in the world, with an estimated 2.26 million new cases observed in 2020. As tumors develop, epithelial cell growth in the lining of the ducts invades the stromal space driving dynamic interactions in the tumor microenvironment (TME) featuring stromal cells and high concentrations of fibrous collagens. Studies have shown that cancer cell secretion of neurotrophic factors changes as the disease progresses contributing to poorer prognosis, linked to an emerging feature of breast tumor innervation (BTI), marked by increased neurite infiltration into tumors. In conjunction, literature evidence suggests that metabolic hallmarks of cancer such as glutamine addiction and Warburg effect may contribute to elevated neurotrophin levels. Yet, it remains unclear how aberrant metabolic behavior of breast cancer cells pertains to BTI. Here, we aim to investigate breast cancer metabolism links to BTI using TME-mimicking three-dimensional in vitro platforms. Our results show that the glutaminase inhibitor significantly reduced optical redox ratio, suggesting effective suppression of mitochondrial OXPHOS activity. In addition, in line with previously established research, minimal expression of neurotrophins was observed from healthy mammary epithelial cells while malignant and DCIS breast cancer cell cultures elevated their secretion of BDNF and GDNF, respectively. Interestingly, glutaminase inhibition significantly reduced neurotrophin secretion by these cells. Our work so far suggests that metabolic rewiring, especially glutamine addiction, may be linked to BTI and warrants further investigation. Our platform will allow for the physiomimetic study of neurotrophin- and metabolism-driven BTI and identify effective therapeutics for breast cancer.
Other Authors: Younghye Song
Tissue-Engineered Models of Lymphatic Drainage in Breast Cancer
Esak Lee and N/A, Cornell University
Although tumor interstitial fluid pressure (IFP) compromises immunotherapies by keeping T cells from infiltrating tumors, the buildup mechanisms of tumor IFP are unclear. Using a vascularized breast tumor model with engineered lymphatic and blood vessels, we discovered that breast cancer tightened lymphatic junctions, impaired lymphatic drainage, and enhanced IFP, while disrupting blood vessel junctions and increasing vessel permeability. Differential contractility in lymphatic and blood endothelial cells led to opposite junction assemblies in normal and tumor conditions; and inhibiting rho-associated protein kinases (ROCK) reversed endothelial contractility, junctions, lymphatic drainage, and vessel permeability. In vivo, ROCK blockade promoted tumor lymphatic drainage, reduced IFP, and enhanced T cell infiltration during anti-PD-L1 immunotherapy without causing metastasis. Our vascularized tumor-on-chip and mouse models reveal that tumor lymphatic normalization potentiates immunotherapy.
Other Authors: Harry Peng
Session 2 – Metastasis
Recapitulating organotropic breast cancer metastasis using a human multi-tissue platform
Gordana Vunjak-Novakovic, Columbia University
A key feature of metastasis is organotropism, where tumor cells disseminate preferentially to specific distal organs. We developed a multi-tissue platform with capability to capture preferential homing of circulating breast cancer cells to distinct tissue niches that they colonize. This multi-tissue model of breast cancer dissemination has two target tissues: bone and lung, that are engineered from human iPS cells and linked by vascular flow containing circulating cancer cells. Uniquely, the organ compartments are separated from vascular flow by endothelial barriers, as in the body, allowing both the long-term maintenance of tissue phenotypes and their communication via cells and secreted factors.
Following infusion of either parental triple-negative breast cancer cells or their bone or lung organotropic progenies, we studied their extravasation across the vascular endothelium and the subsequent tissue colonization. We found that the parental line induced ubiquitous tissue colonization and remodeling, as observed in the whole organism. In contrast, the bone and lung metastatic cell lines showed strong preference for colonization of the bone and lung tissues, respectively. We also found that the bone targeting cells caused osteolysis while lung-targeting cells impaired epithelial function. Finally, we characterized the tissue-specific remodeling of metastatic tissue niches, and identified correlations of the changes in the metastatic tissue niches with the transcriptional changes associated with cancer cell adaptation to bone and lung environments.
The experimental data substantiate that our model system captures both the transcriptional adaptation of cancer cells to their new microenvironments and active remodeling of the tissue niches following metastatic colonization.
Other Authors: n/a
Investigating the Effects of Tensile Strain on Breast Cancer Cell Dormancy Using a Lung-Mimetic Magnetic Actuation Platform
Madison Howard, Purdue University
Breast cancer (BC) often metastasizes to organs experiencing high mechanical stress, including the lungs. Despite this knowledge, the effect that dynamic forces native to the lungs have on early disseminated tumor cells is a currently under explored area of the metastatic cascade. Recent in vitro findings suggest BC cells enter a dormant state in response to tensile strain, yet the mechanisms by which these cells adapt to the dynamic conditions at metastatic sites remain unclear. In this work, we used a lung-mimetic magnetic actuation cell culture platform to apply tensile strain at various amplitudes and frequencies to MDA-MB-231 BC cells. The platform utilizes a fibrillar fibronectin (FN) matrix as the cell seeding substrate, a key component of the early metastatic niche, making it a more physiologically relevant microenvironment. In this study, changes in cellular morphology, proliferation and gene expression between cyclic stretching, constant stretching and static conditions were evaluated. We found applying tensile stretch decreased overall cell metabolic activity but did not affect cell viability, indicating the cells entered a dormant state. Applying tensile stretch to the FN substrate changed the cell morphology to be consistent with previously reported senescent cells. Further, RNA sequencing of the cells after four days of stretching identified differentially expressed genes compared to the static control group. This effect was amplified in the constant stretch group, where additional DEGs were identified. Additionally, we utilized fluorescent reporter cells to compare DNA damage accumulation between the different loading conditions. Based on our RNA sequencing results, which showed an increase in Insulin-like growth factor-binding protein 3 (IGFBP3), we hypothesize DNA damage accumulation could be causing this dormant state in response to tensile stretch. These findings seek to elucidate mechanisms of BC dormancy and mechanical adaptation, offering new insights into how metastatic cells survive within the dynamic conditions of the lung microenvironment.
Other Authors: Luis Solorio
A colorectal cancer-on-a-chip model to study mechanical forces and disease progression
Shannon Mumenthaler and Curran Shah, Ellison Institute/USC
Here, we present a patient-derived colorectal cancer organ-on-chip (CRC-OOC) platform that models the tumor microenvironment, including tissue-tissue interfaces and mechanical forces, to study CRC progression. Tumor organoids or cell lines with distinct molecular profiles were fragmented and seeded into the top channel to form an epithelial layer, while human endothelial cells seeded in the bottom channel formed a tube-like vasculature, creating an epithelial:endothelial interface separated by a porous membrane.
Within 2–3 days, the system developed an intact epithelial barrier, verified by permeability assays and ZO-1 tight junction staining. Inter-patient heterogeneity was evident through differences in epithelial morphology, gene expression, and drug response profiles analyzed via live-cell imaging and multi-omics analysis. The platform's ability to mimic intestinal mechanical forces, including fluid shear stress and peristaltic-like cyclic strain, demonstrated that these forces significantly influence tumor behavior. Specifically, peristaltic-like motions induced epithelial-to-mesenchymal transition (EMT) and enhanced tumor invasion into the endothelial layer, as shown by confocal imaging. Mechanistic studies revealed that PIEZO1 mediates the tumor response to cyclic strain.
This CRC-OOC model underscores the critical role of mechanical forces in shaping the tumor microenvironment and provides a robust tool for investigating CRC progression, heterogeneity, and therapeutic responses.
Other Authors: N/A
Ovulation as a risk factor for high grade serous cancer
Joanna Burdette and Angela Russo, Univ of Illinois-Chicago
The lifetime number of ovulations is a risk factor for developing ovarian cancer. High grade serous cancers can arise from the fallopian tube epithelium. Our study was designed to investigate how ovulation may impact fallopian tube carcinogenesis. We developed a microfluidic chip that can support ovulation of murine ovaries under the influence of exogenous gonadotropin stimulation. Secreted factors from the ovulating ovary chamber are perfused to a second paired chamber that supports the growth of primary human fallopian tube tissue at an air liquid interface. The platform supports 15 paired interacting tissues. Ovaries can be stimulated to ovulate over a 28 day or abbreviated 14 day cycle and produce the steroid hormone estradiol during the follicular phase and progesterone during the luteal phase. The secreted factors produced by the ovary were collected in the microfluidic chamber effluent over the course of a menstrual cycle and subjected to proteomics to profile proteins that interact with the fallopian tube tissue over the course of ovulation. Primary human fallopian tube tissue was subjected to GeoMx DSP spatial transcriptomics to capture differential mRNA expression before and after ovulation in the ciliated and secretory fallopian tube epithelium and revealed repression of the thioredoxin-interacting protein (TXNIP) transcript that encodes a putative tumor suppressor and regulator of metabolism. TXNIP expression has been shown to be negatively regulated by IGF1. Interestingly, IGF1 was present in the proteomics from the secreted ovarian products collected on chip providing a mechanism for the repression.
Other Authors: Jonathan Coppeta
Session 3 – Drug Discovery / Screening
Novel Vascularized Micro- physiologic system of Peritoneal Carcinomatosis
Maheswari Senthil and Dr. Christopher Hughes, University of California Irvine
Background:
Advances in the management of Peritoneal carcinomatosis (PC), the deadliest metastasis in gastrointestinal (GI) malignancies has been impeded by lack of appropriate in vitro tumor models that recapitulate the peritoneal tumor micro-environment (TME). The peritoneum consists of a mesothelial layer and sub-mesothelial stroma and is separated from the systemic circulation by the blood peritoneal barrier, which limits penetration of systemic therapy into peritoneal metastases. Here we report, the development of a 3D Vascularized Micro-Peritoneum (VMP), an in vitro PC model based on our vascularized micro organ, "vasculature-on-chip" platform.
Methods:
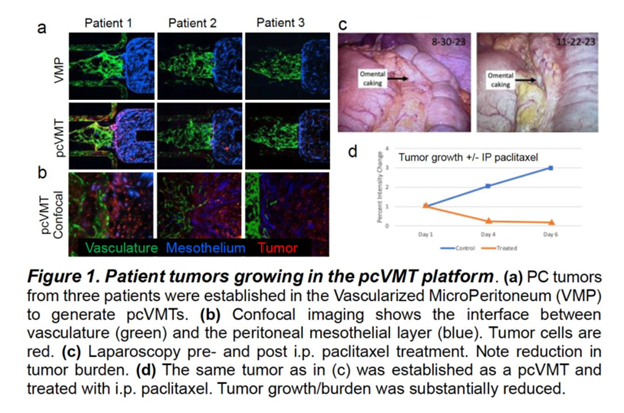
We designed a two-chamber device to recapitulate the peritoneal cavity and sub-mesothelial stroma and barrier. The VMP target tissue was created by coating the “peritoneal surface” with laminin followed by seeding normal human mesothelial cells. The sub-mesothelial stroma was developed with endothelial and stromal cells to create a perfused vascular network (Figure 1a). Then, patient-derived PC tumor cells were introduced into the peritoneal cavity where they adhered to the mesothelial cells and proliferated into PC over 3-5 days.
Results: We have successfully established PC tumors from patients (n=8) in the VMP. Figure 1a shows patient-derived gastric pcVMT tumors (n=3) treated with intraperitoneal (IP) paclitaxel. Response in the model correlated with in vivo treatment response seen on laparoscopy (Figure 1c and 1d).
Conclusions: The establishment of the pcVMT model provides an incredible opportunity to understand the interplay of tumor microenvironment, sub-mesothelial stroma, tumor vasculature and the complex extracellular matrix (ECM) in PC and develop novel treatment approaches.
Other Authors: Aaqil Khan MS
Investigating Targeted Therapeutics in Diffuse Intrinsic Pontine Glioma Using Tumor-Tissue Analogs
Meenakshi Upreti and Jennifer Ritchie, Children's Hospital Los Angeles
Diffuse intrinsic pontine glioma (DIPG) is an incurable pediatric brain tumor with a two-year survival rate of 10%. The brainstem location makes these tumors non-resectable and resistant to therapy with radiation improving survival by about two months. Targeted cancer therapeutics offer an innovative approach to treating DIPG. However, identifying targets specific to DIPG remains a challenge. Models that better represent the tumor microenvironment and stromal influence on tumor protein expression, are needed to advance this approach. We have devised an in vitro disease model for DIPG via self-assembly of select non-malignant stromal and malignant cell types of human origin including microvascular brain endothelial cells, microglia, and tumor cell lines to form 3D tumor tissue analogs (3D-TTA). A comparison of transcriptomic (RNA Sequencing) and proteomic (Mass Spectrometry) expression between the DIPG cells in the 3D-TTA and tumor cell only cultures identified 14 proteins/genes that were differentially expressed on the DIPG cells under stromal influence, seven of which were expressed in plasma membrane: Chloride intercellular channel protein 1 (CLIC1), collagen VI family (COL6A), Phospholipid scramblase 1(PLSCR1), vascular cell adhesion molecule-1 (VCAM-1), integrin αVβ5 (IαVβ5), galectin-1, and galectin-3.
Chlorotoxin-conjugated iron oxide nanoparticles (NP-CTX) as a targeted therapeutic was utilized to evaluate for specificity and internalization. The targeting ability of NP-CTX in four of the stroma induced membrane proteins VCAM-1, IαVβ5, galectin-1, and galectin-3 was validated. Neuropilin-1 and matrix metallopeptidase-2 (MMP-2) served as positive controls. Here we demonstrate the utility of tumor tissue models in the development of therapeutic strategies using functionalized nanoparticles. Efforts underway are investigating targeted and cellular immuno-therapies in this model.
Other Authors: Ahmed Mohamed, Jacob Al-Husseini, Aleksey Lyzlov, David Koos PhD, Rex Moats PhD, Peter Chiarelli MD-PhD,
Patient micro-organoids driving clinical drug development decisions
Xiling Shen, MD Anderson
With the advent of FDA Modernization Act 2.0, patient-derived xenografts (PDX) and organoids (PDO) have been increasingly used for pre-clinical drug development. Here we used droplet emulsion to rapidly generate thousands of micro-organoids (MOs) from low-volume patient tissues to accelerate clinical development and make clinical trials ‘smarter’. MOs capture original stromal cells and allow T cell penetration, providing a clinical assay to assist clinical trial decisions for various immunotherapies. We further demonstrate an ultra high-throughput screening platform that provides a “virtual clinical trial” to assess the therapeutic window by determining drug efficacy and toxicity.
Other Authors: N/A
3D Bioprinting for Modeling the Brain and Glioblastoma
- Shrike Zhang, Brigham and Women's Hospital, Harvard Medical School
The dynamic tumor microenvironment (TME) where cells continuously communicate, migrate, and react to each other and the signals that are secreted, is critical for inducing tumor progression and aggressiveness of most forms of cancer. We have special interest in glioblastoma (GBM) that displays a dynamic and complex TME for which we have developed the necessary tools to dissect it, understand it, and have a positive impact on its treatment. As such, it is necessary to understand the underlying biology in a dynamic and relevant environment. With various degrees of limitation pertaining to currently available in vitro and in vivo models, in this funded project, we aim to leverage our expertise to optimize a unique three-dimensional (3D) human mini-GBM model through the utilization of a light-based bioprinting technology and taking advantage of primary neuronal, vascular, and GBM cells, to more precisely replicate the brain TME in human patients. It is anticipated that, construction of an in vitro 3D human mini-GBM model mimicking not only the cellular compositions but also the extracellular matrix (ECM) properties and importantly, tissue architecture of its in vivo counterpart, will allow us to precisely assess proliferation, migration, and transformation of GBM cells, similar to those already proven in ex vivo GBM organotypic cultures but at much higher availability and throughput for potential drug screening in the future. This presentation will provide the general overview of the technologies that we use for constructing the in vitro mini-GBM model as well as project progress up to date.
Other Authors: Alfredo Quiñones-Hinojosa
Session 4 – TME 2
Deconstructing a complex microenvironment with geometrically structured microtumours
Kris Kilian and Thomas Molley, UNSW Sydney
The tumor microenvironment (TME) consists of a complex assortment of multiple cell types and dynamic extracellular matrices. Model systems that mimic aspects of the TME are useful tools for probing signal transduction underlying progression. In this presentation I will demonstrate how mimicking the biophysical and biochemical attributes of the TME in spatially addressable assays can be used to better probe cancer processes. Hydrogel micropatterning can be used to coordinate the interactions of cancer cells and stroma, where confinement on deformable substrates triggers partitioning of different cell types in ways that mimic organisation in vivo. Regions of perimeter curvature are shown to activate fibroblasts to a contractile state, thereby leading to enhanced matrix deposition and corralling of cancer cells to the center. Using a geometric templating approach brings these assays into 3D, with the possibility of directing bi-directional cross talk with spatiotemporal control. Leveraging biofabrication can further increase the complexity by allowing cancer spheroids, stromal cells and vessel structures to be integrated within a single system. The fabrication of multiple aspects of the TME allows better control over features responsible for progression, thereby providing improved tools for fundamental studies and drug development.
Other Authors: Giulia Silvani, Sylvia Ganda, Yiling Liu, Chantal Kopecky
Fluid Assisted Transformation and Dissemination of Fallopian Tube Epithelial Cells
Geeta Mehta and Analisa DiFeo, University of Michigan
High grade serous ovarian carcinoma (HGSC) is the most common type and most lethal gynecologic cancer due to its late-stage diagnosis. One of the cells of origin of HGSC reside in the fimbria of the fallopian tube. The cells lining the lumen of the fallopian tube are stimulated with varying levels of shear stress, which can vary in magnitude in the different phases of the menstrual cycle, pre- and post-menopause under normal healthy conditions. The role of shear stress in the fallopian tubes is well recognized for the transport of gametes and embryo. However, the role of fluid flow and shear stress in the fallopian tube epithelium remains unexplored in the context of premalignant fallopian tube epithelial secretory cells (FTSEC), that may undergo early precursor escape and result in HGSC initiation. Our central hypothesis is that shear stress stimulation transforms the FTSEC in the fimbria to activate EMT pathways and results in their dissemination in the peritoneum. We are working towards the following Aims to address the scientific knowledge gap and the central hypothesis: 1) Characterize fluid assisted transformation and dissemination in FTSEC within custom-built microphysiological systems. 2) Determine the bulk viscoelastic and mechanical properties of the healthy human fallopian tubes and their local heterogeneity. 3) Identify and validate the mechanotransduction pathway governing the transformation of FTSEC under shear stress stimulation. By completing this work, we expect to develop new knowledge about molecular mechanisms involved in the mechanobiology of shear stress driven programming of FTSEC.
Other Authors: Raneem Ahmad, Isha Bhorkar, Nina Treacher, Eric Horst, Analisa DiFeo, Mike Solomon, Ron Larson
Effects of matrix mechanics and hypoxia on glioblastoma metabolism
Stephanie Seidlits, University of Texas at Austin
Glioblastoma (GBM) is a uniformly lethal brain cancer due to poor treatment response and high rates of recurrence. The unique brain environment is believed to play a key role in GBM progression and malignancy, as tumors very rarely metastasize to other organs. To better understand the effects of the tumor microenvironment on treatment resistance and disease recurrence, we previously characterized the effect of varying matrix stiffness on GBM cell proliferation, invasion, and metabolism in 3D brain-mimetic hydrogels mimicking the stiffness of peritumoral brain tissue or the tumor bulk (Sohrabi, et al. 2023). While these observations were made using cultures maintained at 21% O2, the O2 concentration in brain tissue is lower and hypoxia is known to affect cell metabolism and GBM progression. For the current study, we cultured GBM spheroids in 3D matrices with defined elastic moduli in 21% or 1% O2 to identify how these effects interact to determine GBM behavior. GBM cells cultured in soft matrices migrated significantly more than cells cultured in stiff conditions, regardless of O2 levels. GBM cells derived from multiple patient tumors and cultured in soft matrices were more invasive in 1% O2 than in 21% O2. After 24 hours, cells in stiff matrices cultured in either O2 condition had increased activity across proliferation and survival pathways. However, by day 7 proliferation-related signaling was downregulated, except for the Src pathway which remained consistently upregulated in stiff matrices. We identified distinct trends in metabolite production, in particular polyamine synthesis, associated with stiffness and oxygen conditions.
Other Authors: Mollie Harrison, Talia Sanazzaro, Yuan-I Chen, Shuxin Dong, Daylin Morgan, Alireza Sohrabi, Hsin-Cheh (Tim) Yeh, Amy Brock, Alessia Lodi, Stephanie Seidlits